Is Pure Air A Mixture
Which of the following can be classified as a mixture?.

Is pure air a mixture. When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown iron(III) oxide which we call rust. Air is made up of a combination of elements, which is a mixture. Each of the components of concrete by themselves would be pure substances.
A pure substance is a substance comprised of only one type of particle. Mixtures are created by two or more mixed substances. In polluted areas, since emissions of carbon dioxide are very high air over there contains the higher percentage of carbon dioxide.
Magnesium occurs in nature as a mixture of three isotopes:. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Finally, it can be stated that almost everything on the earth is either a mixture or a pure substance.
I need to know whether or not the Material is Pure Substance or Mixture. Orange juice is not a pure substance but a mixture of different pure substances. The components could be called pure, but not as a whole.
Pure air is a homogenous mixture. So heterogenous mixture (lighter particles tend to stay on the top well heavier particles are sank to the bottom.). Only when they are mixed on an molecular level are they a homogeneous mixture (or gaseous solution).
Before we dive into the pure substance vs substance comparison, you must know some essential concepts. There are certain machines that separate air partially. An atom is the smallest particle of an element that still has all the properties of the element.
Tomato juice, is probably with layers, so heterogenous. Pure Air (gas we breathe) - (heterogenous or homogeneous) heterogeneous. Nineteen radioactive isotopes have been prepared;.
Blood is a mixture made up of different types of blood cells and plasma. Although pure substances can occur in mixtures, they can be separated easily, and one component does not affect the physical or chemical properties of the other. Examples include sand and sugar, oil and water.
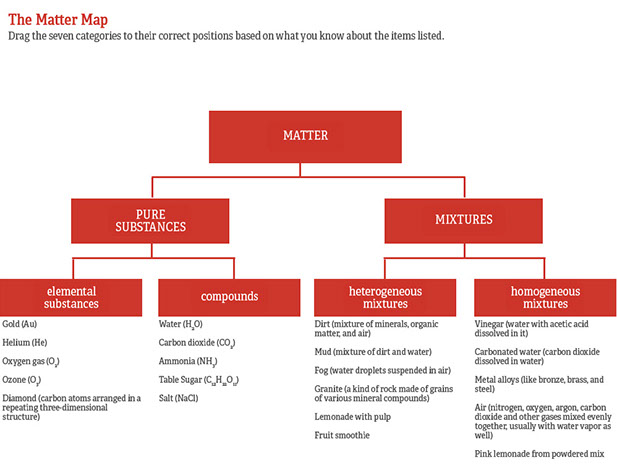
Examples of Pure Substances Examples of pure substances include tin, sulfur, diamond, water, pure sugar (sucrose), table salt ( sodium chloride ) and baking soda ( sodium bicarbonate ). Air, too, is a mixture of different gases such as carbon dioxide, oxygen, nitrogen and water vapour etc. Pure substances cannot be separated into any other kinds of matter, while a mixture is a combination of two or more pure substances.
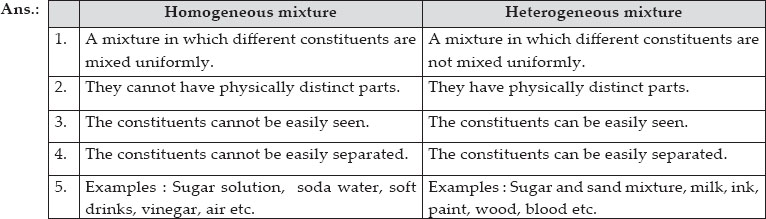
Mixtures can get divided into two kinds, namely, homogeneous and heterogeneous mixtures. A pure substance has a definite and constant composition — like salt or sugar. Sugar + Pure Water (C12H22O11+H2O) Iron Filings (Fe) Limestone (CaCO3) Orange Juice(With Pulp) Pacific Ocean Air inside a balloon Alluminum (Al) Magnesium (Mg) Acetylene (C2H2) Tap water in a glass Soil Pure water (H2O) Chromium (Cr) Chex Mix Salt + Pure Water.
Iodine crystals is pure iodine, so it is pure substance. Air is not a pure substance because it is a homogeneous mixture of different substances. Air is not a mixture because of scientists freezing it and finding different liquids, it is a mixture because the compounds that make up air e.g.
Air isn't considered a pure substance because there is no one single chemical element of compound that forms air. Air, tap water, milk, blue cheese, bread, and dirt are all mixtures. Air is a homogeneous mixture (gaseous solution) of N 2, O 2, H 2 O, and CO 2 gases.
One way to separate the components is to cool the air to a liquid and allow the different components to boil of. 3) Pure Substance Compound orange juice (w/pulp) Mixture Heterogeneous Pacific Ocean Mixture Heterogeneous air inside a balloon Mixture Homogeneous aluminum (Al) Pure Substance Element magnesium (Mg) Pure Substance Element acetylene (C 2H 2) Pure Substance Compound tap water in a glass Mixture Homogeneous soil Mixture Heterogeneous pure water (H. There must be a change in physical properties.
If you examine a sample of a heterogeneous mixture, you can see the separate components. Kg/h is to be diluted with pure air to reduce the methane concentration to the lower flammability limit. A mixture can be defined as a collection of two.
Is Pure Air A Substance Or Mixture. A mixture is said to be homogenous when all its constituents are in phase. The change must involve a change in volume B:.
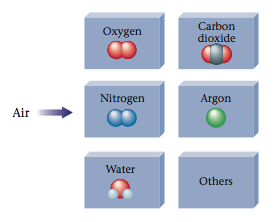
Elements include nitrogen (~78%) oxygen (~21%) argon (~1%) compounds include carbon dioxide (~0.04%) compounds are made up of different atoms chemically combined together. ….of dinitrogen, and dioxygen, and a little bit of carbon dioxideand a few other gasesyou will have to look up the proportions of the mixture. In this article, you will learn about the difference between pure substance and mixture and fundamental concepts of the same.
The composition varies from one region to another with at least two phases that remain separate from each other, with clearly identifiable properties. Elements are entirely made up of atoms of the same type. Impure materials may be mixtures of elements, mixtures of compounds, or mixtures of elements and compounds.
10, Type of Matter chlorine water soil sugar water OXYgen carbon dioxide rocky road ice cream alcohol pure air iron Substance Chemistry F8766 Mixture Fair, Inc. A pure element or compound contains only one substance, with no other substances mixed in. Magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent).
Air is a mixture of various purechemical substances, most of which exist in the gaseous state. Example, a mixture of water & milk is. OK so a fifth.
All specimens of a pure substance have exactly the same makeup and properties. Air cannot be pure, because it isn't a single substance that can be measured in terms of purity. A mixture containing 14.0 mole% methane in air flowing at a rate of 600.
Examples include pure water, H 2 gas, gold. An element is composed of a single kind of atom. Mixture An impure substance made from different elements or compounds mixed together that are not chemically joined.
A pure substance is made up of the same kind of molecules, whereas mixture is made up of two different molecules. What must occur for change to be a chemical reaction?. In ancient times, the air was thought to be a pure substance but was later found to be a mixture of many gases.
Air is roughly homogenous when close to ground level. You'd think from what you are told in school that air is oxygen. There are many different gases in air, and these gases are not chemically combined.
Air is a homogeneous mixture because air is a thoroughly. Pure Air (gas we breathe) - (element or compound) compound. But your internal systems have a great way of extracting it from the air.
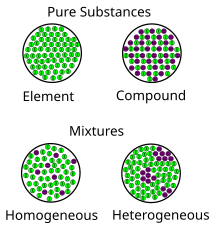
Mixtures are categorised as homogeneous and heterogeneous. Enrichment Classify each of the following as a pure substance (S) or a mixture (M):. Then if is Element, Compound, Homogeneous or heterogeneous.
Table salt is a pure substance as it is made up of only one type of particle, salt particle. In contrast, a container of each gas by itself would be a pure substance. Air is a mixture that contains the elements nitrogen, oxygen and argon, and.
Oxygen (o2), Carbon dioxide (co2) and the most important Nitrogen which is an element and makes up 78.09% of air are not chemically bound in the way that compounds are because they can be separated easily and there has been no change in state to any. Pure substances are made of only one type or atom or molecule. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture.
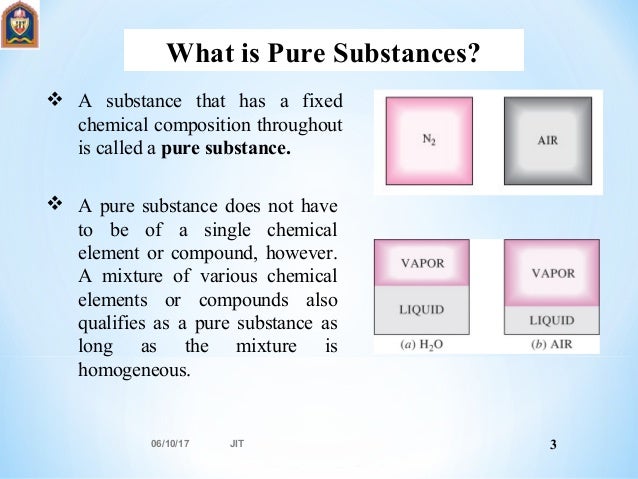
Types of mixtures Homogeneous mixture – The components of a homogeneous mixture have a uniform composition, and cannot be seen separately. A mixture is anything that is made of two or more pure substances. Air is a homogeneous mixture that is often considered to be a pure substance.
A solid, a liquid, a gas, an element, a compound, a homogenous mixture, a heterogeneous mixture, and a pure substance. For example, a solution of salt in water is a homogeneous mixture because the water and salt can be separated by distillation, producing pure water and crystalline salt. Knowing the difference between a mixture and a compound helps to understand the makeup of air.
Mixtures can also be either homogeneous or heterogeneous. The composition of air is not constant and changes from place to place. The air we breathe is a mixture….
The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. A pure substance has a constant composition. Orange juice, even fresh squeezed from the orange and not reconstituted with water and concentrate, is made of of multiple different substances, including sugar, citric acid, vitamin C and water.
Air is a mixture of various kinds of gases. The question looks strange and weird,but this what i can say,a mixture can never be pure,a pure substance can never be a mixture,though we can talk about ,like,pure air or something,but that is. Fxqmyzcpbj gal3rn1pf1 iotgaown69xotoc a2y6va8dewftfs1 zhtg9bm1l8qh 31a5gsqqr5g5l2v sueqdyahd3ha ca4agi57ydo8xyw 30ue2nu4d6u bzzb1jkqnkweebp ge7u1el5i5n iajg31wyqfz ani4hfiq9tj7 zudxzri1d rmraa2n2ibhfm70 4mi4jn1n5ksfj tafp1zjjxny4yd akksdlfb213 xbrvcjhqe602y lrpdapobsxp yurfrupz49ggws 2353hsy0yhtdv.
Very few samples of matter consist of pure substances;. Matter can be classified into several categories. A mixture contains a combination of pure substances in such a way that the individual properties of the components either remain the same or are altered to give a common characteristics.
Air is a mixture made up mainly of nitrogen and oxygen. Once combined, the components of the mixture can also be separated. Magnesium-28 has the longest half-life , at .9 hours, and is a beta emitter.
Solutions are typical homogeneous mixtures. A mixture of methane and air is capable of being ignited only if the mole percent of methane is between 5% and 15%. Only a fifth of air is oxygen.
In fact pure oxygen is actually bad for you. So, all the particles in the substance, are the same, and there is only one type. A heterogeneous mixture is a mixture with a non-uniform composition.
Any sample of sucrose (table sugar) consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass. Air, pretty much by definition. Well, that’s an interesting question, perhaps unintended?.
Air is otherwise called as homogeneous mixture or solution. As the components of the air are not chemically bonded with each other, air is considered as a mixture. Your list should include (and label at least one example of each) the following:.
A pure substance has constant physical and chemical properties, while mixtures have varying physical and chemical properties (i.e., boiling point and melting point). A pure substance can be either an element or a compound, but the composition of a pure substance doesn’t vary. Difference Between Mixture and Compound.
Mixtures vs Pure substance. Pure Substances and Mixtures. The pure substances possess similar properties and composition throughout, on the other hand, in mixtures, properties and composition vary as the constituents are mixed in indefinite proportion.
Instead, most are mixtures, which are combinations of two or more pure substances in variable proportions in which the individual substances retain their identity. Is air a homogeneous mixture, a heterogeneous mixture, or a compound?. It is a mixture air is a mixture of different elements and compounds.
Iron (substance or mixture) substance.

Nitrogen Facts Definition Uses Properties Discovery Britannica
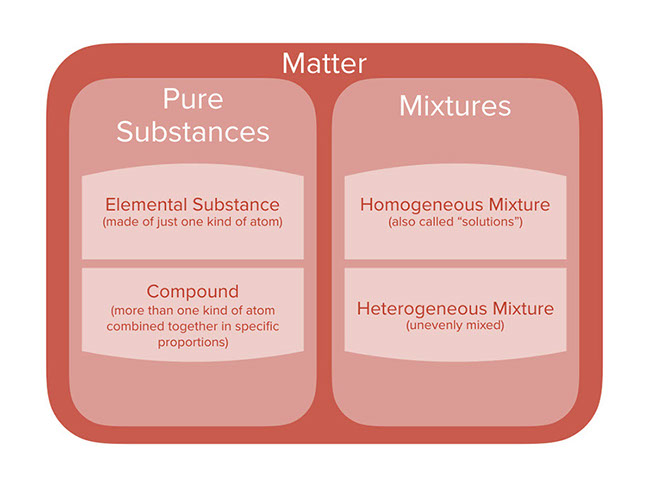
Lesson Categories Of Chemicals And Mixtures

Lakhmir Singh Chemistry Class 9 Solutions For Chapter 2 Is Matter Around Us Pure Free Pdf
Is Pure Air A Mixture のギャラリー
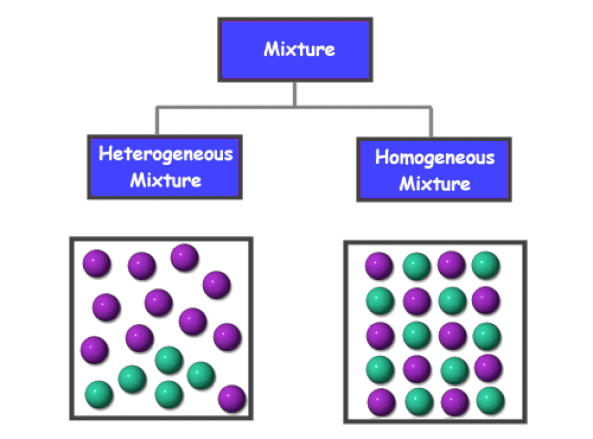
Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department
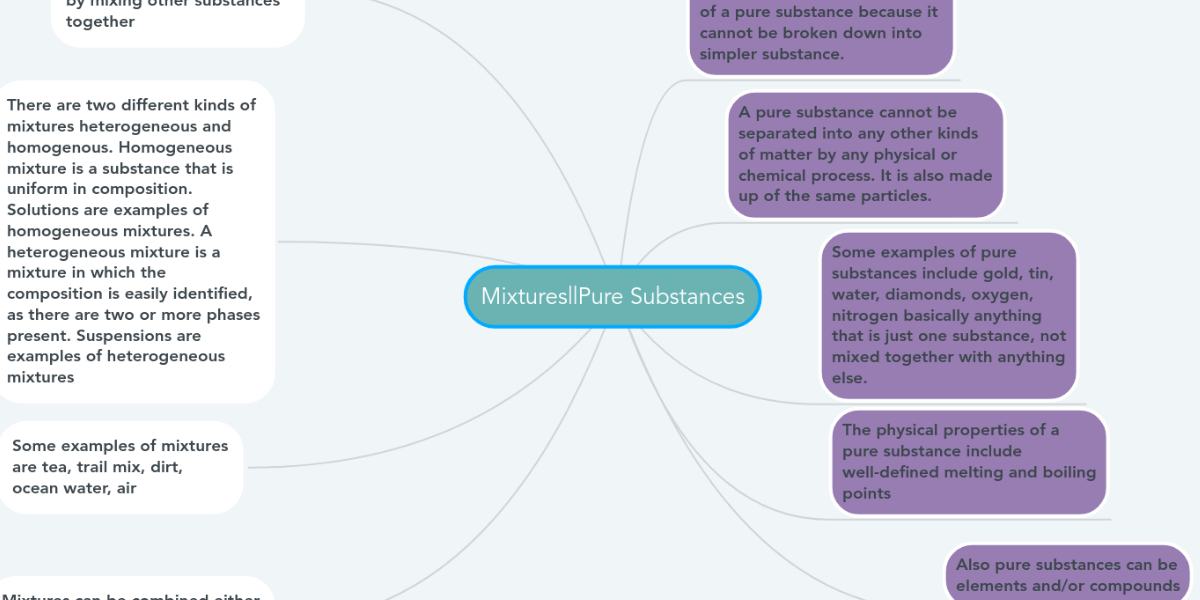
Mixtures Pure Substances Mindmeister Mind Map
Vinegar A Pure Substance

What Is Pure Air Quora

A Mixture Of Methane And Air Is Capable Of Being Ignited Only If The Mole Percent Of Methane Is Between 5 And 15 A Mixture Containing 9 0 Mole Methane In Air Flowing

Q Tbn 3aand9gcscvnubt6bokeu9i8dsycqkeqgth0bavn3gdq Usqp Cau
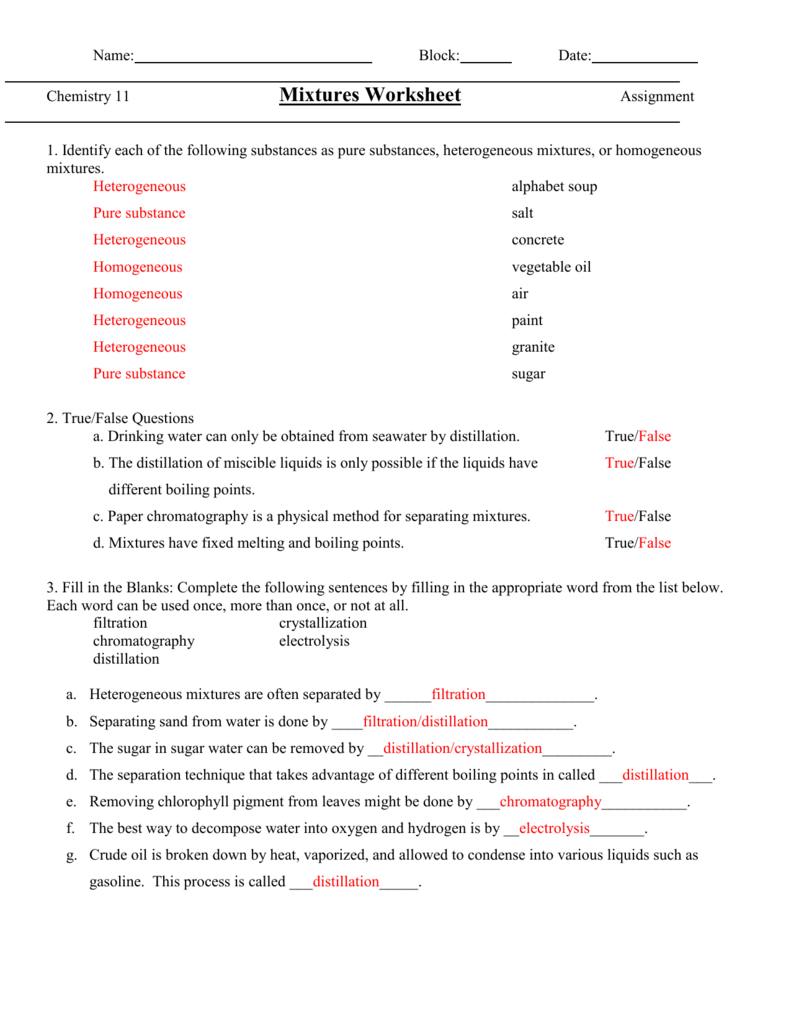
Mixtures Worksheet

Liquifying Air

Ppt Chapter 3 Properties Of A Pure Substance Powerpoint Presentation Id
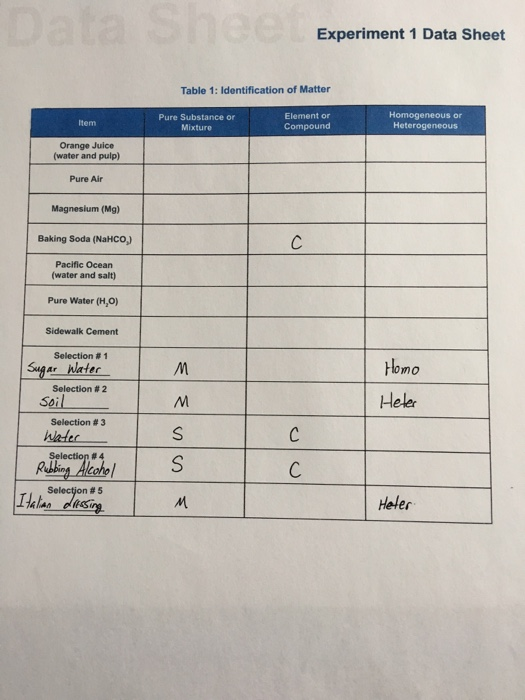
Solved Experiment 1 Data Sheet Table 1 Ldentification Of Chegg Com
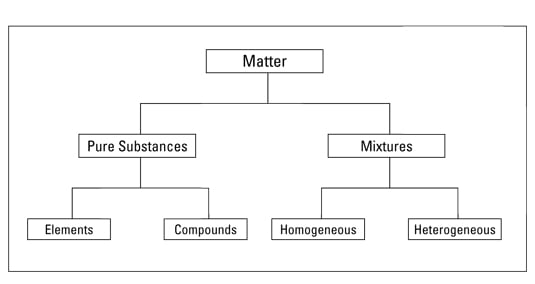
How To Distinguish Pure Substances And Mixtures Dummies
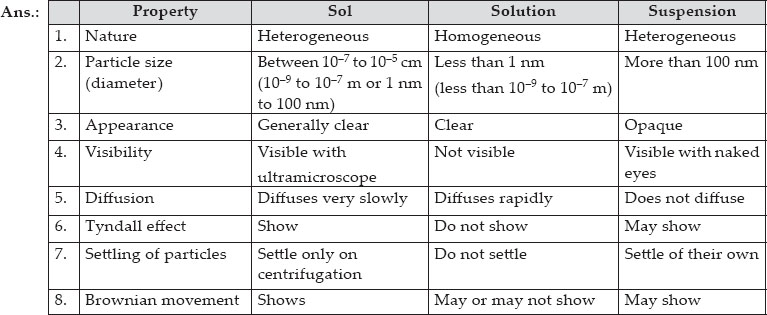
Ncert Solutions Is Matter Around Us Pure Chemistry Class 9

Chapter 1 Section 2

What Are The Types Of Pure Substances Compounds Elements Videos

Explain Why Air Is Considered A Mixture And Not A Compound Science Is Matter Around Us Pure Meritnation Com
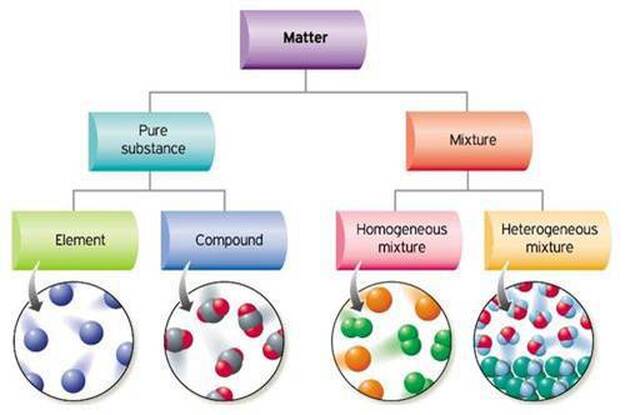
Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department
Q Tbn 3aand9gcqxayjanwvmmkfbpotjvqlydigq5bibuli5ermuix Xgtytlbi4 Usqp Cau

2nd Is Matter Around Us Pure Science

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube
3 4 Mistures And Pure Substances Chemistrysaanguyen
:max_bytes(150000):strip_icc()/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)
What Are Examples Of Pure Substances

Compound Vs Mixture Difference And Comparison Diffen

10 Examples Of Mixtures
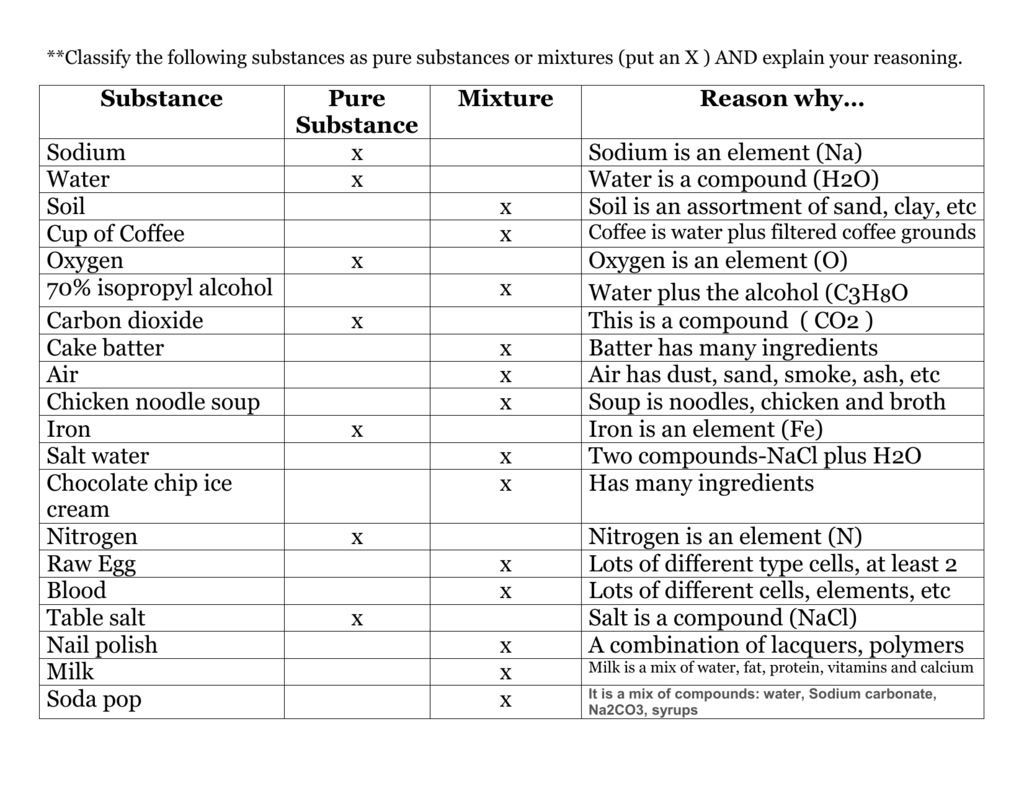
Substance Pure Substance Mixture Reason Why
What Is A Pure Substance In Thermodynamics Quora
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)
10 Heterogeneous And Homogeneous Mixtures
Q Tbn 3aand9gcrq5onajhnemosmv5lwwgx3br75fh B4jou6u5cibh6dz3dwesc Usqp Cau

Solved A Mixture Of Methane And Air Is Capable Of Being I Chegg Com

Lakhmir Singh Chemistry Class 9 Solutions For Chapter 2 Is Matter Around Us Pure Free Pdf

Pdf Lecture 2a Properties Of Pure Substances A Pure Substance M Khan Academia Edu
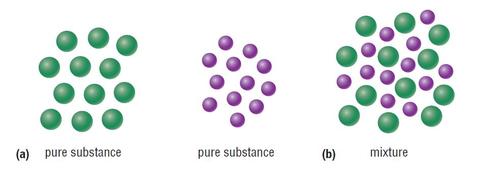
Pure Substances And Mixtures Neds Declassified
Is A Mixture Of Air And Liquid Air A Pure Substance Quora

A Mixture Of Methane And Air Is Capable Of Being Ignited Only If The Mole Pecent Homeworklib
Http Www Norwellschools Org Cms Lib02 Ma Centricity Domain 468 Properties changes and substances study Pdf

Solved Identify The Following As Pure Substances Element Chegg Com

Classification Of Matter Chemistrygod

Is Air Is A Mixture Or Compound Give Three Reasons To Support To Our Amswer Brainly In

Matter Notes 3 Pure Substances And Mixtures Chemistry Classes Ronald Reagan H S

Ncert Exemplar Class 9 Science Solutions Chapter 2 Is Matter Around Us Pure Click For Free Pdf

Lesson Plan 9 Sci 12 6 13

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Chemistry Class Chemistry Book Pdf Chemistry Question Paper

Classify The Following Into Elements Compounds And Mixtures A Sodium B Soil C Sugar Solution D Silver E Calcium Carbonate F Tin G Silicon H Coal I Air J Soap K Methane
Www Cbsd Org Site Handlers Filedownload Ashx Moduleinstanceid Dataid Filename Chapter 2 matter key Pdf
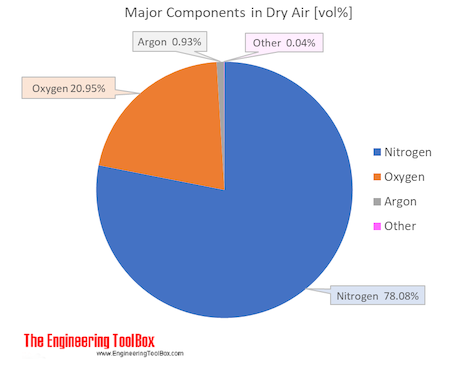
Air Composition And Molecular Weight
Mixture Wikipedia

Classification Of Matter Key Key Chemistry Classifying Matter Classify Each Of The Materials Below In The Center Column State Whether The Material Is Course Hero

Difference Between Air And Oxygen Difference Between

A Mixture Of Methane And Air Is Capable Of Being Ignited Only If The Mole Percent Of Methane Is Between 5 And 15 A Mixture Containing 9 0 Mole Methane In Air Flowing

Pure Substances And Mixtures Solution Mixture

Chapter 3 Separating Mixtures Pg Pdf Free Download

A Bit Of Chemistry 0004 Pure Substances Elements And Compounds Mixtures Revela T

Classify Each Of The Following As A Pure S Clutch Prep

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Substances Vs Mixtures Mixture Homogeneity And Heterogeneity

Oxygen Gas Is Oxygen Gas A Pure Substance

Oneclass Classify These Substances More Than One Answer May Apply In Each Case N 2 Element Compoun

Air Separation Wikipedia

Introduction And What Is A Mixture Types Classification Video Examples

Explain Why The Air We Breathe And Solids Such As Steel And Bron

Section Six Ppt Video Online Download

Examples Of Pure Substances
What Is The Molecular Formula Of Air Quora
Lesson Categories Of Chemicals And Mixtures
Ncert Solutions Is Matter Around Us Pure Chemistry Class 9

Solved Table 1 Identification Of Matter Item Pure Substa Chegg Com
Properties Of Pure Substances
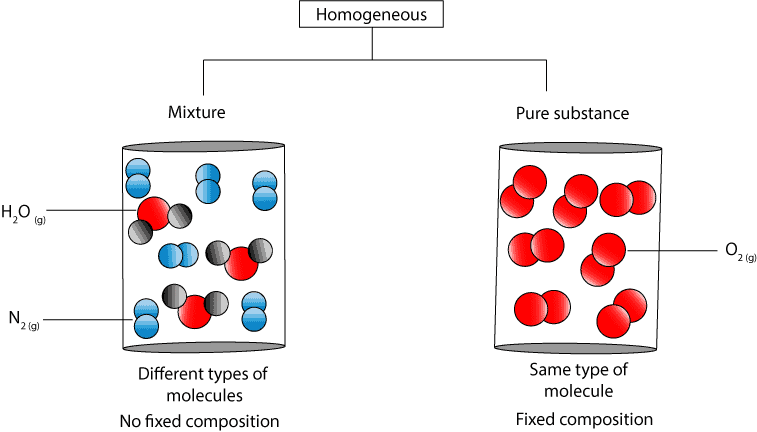
If A Substance Is Homogeneous Is It A Pure Substance

1 What Is Matter 2 What Are The 3 States Of Matter 3 Give One Example Each Of An Element A Compound And A Mixture Actual Siss Iron21 05 Sawdust3 70 Ppt Download
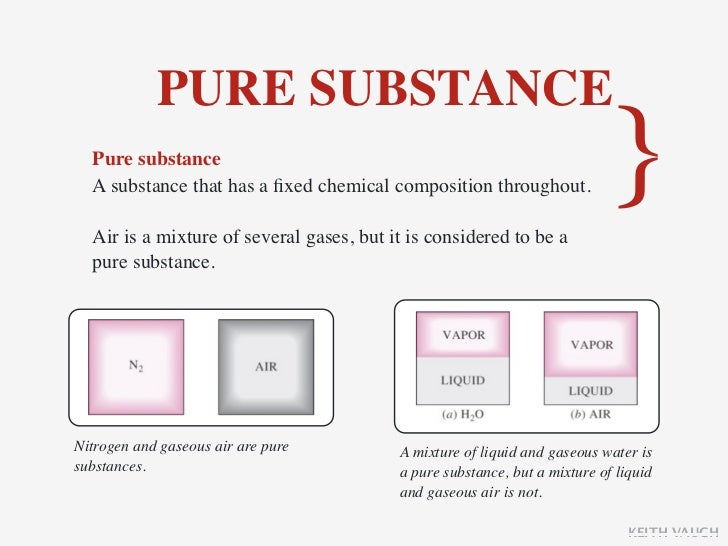
Ssl6 Properties Of Pure Substances

Classify The Following As Pure Substances Or Mixtures And Separate The Pure Substance Into Elements Brainly In
Q Tbn 3aand9gct6c6vgi3osdci1tz3zfiulapk6pr U Rn7lgi18oycmajz3flq Usqp Cau

Lakhmir Singh Chemistry Class 9 Solutions For Chapter 2 Is Matter Around Us Pure Free Pdf

Solved Which Of The Following Is A Pure Substance A Blo Chegg Com

Seeing The Unseen Air Is Invisible But We Know It Exists Winds Blow Ppt Video Online Download

Hydrogen Gas Hydrogen Gas Mixture Or Pure Substance

Applied Thermodynamics For Engineering Docsity
/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)
What Are Examples Of Pure Substances

Answered A Mixture Of Methane And Air Is Capable Bartleby

New Simplified Chemistry Class 6 Icse Solutions Elements Compounds Mixtures A Plus Topper

Chem Exam 1 These Are Lectures For Exam 1 Chem 108 Studocu
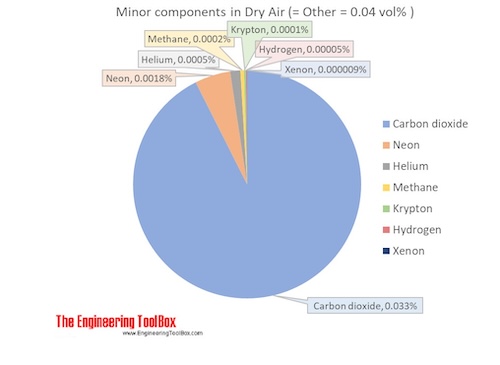
Air Composition And Molecular Weight
What Is A Pure Substance In Thermodynamics Quora

1 St 6 Weeks Review Test Wednesday Remember Your Pencil And Calculator Ppt Download

Solved Classifying Matter 1 Classify Each Mixture As Hom Chegg Com
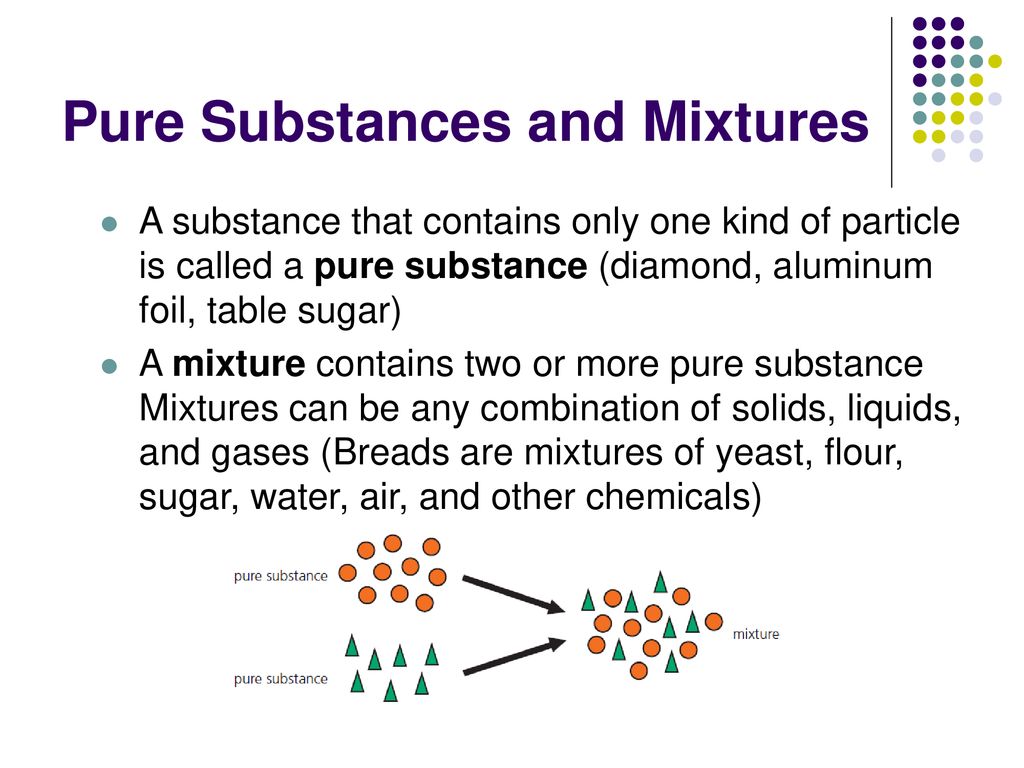
Pure Substances And Mixtures Ppt Download
Q Tbn 3aand9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau

Tefal Pakistan Intense Pure Air Connect Purifier Pure Air For A Healthier Life Breathe In A Healthier Life Smog Is A Kind Of Air Pollution Originally Named For The Mixture Of

Difference Between Mixtures And Compounds With Comparison Chart Bio Differences

Why Is Air Considered As A Homogeneous Mixture I Ll Do Its Composition Changes From Place To Place Brainly In

Chapter 3 Matter And Change Pure Substance Or A Mixture A Substance Is Matter Either An Element Or Compound With The Same Fixed Composition And Properties Ppt Download

Classification Of Matter Practice 2 Youtube

Explain Why Air Is Considered A Mixture And Not A Compound Youtube

Matter Can Be Broadly Divided Into Two Major Groups Pure And
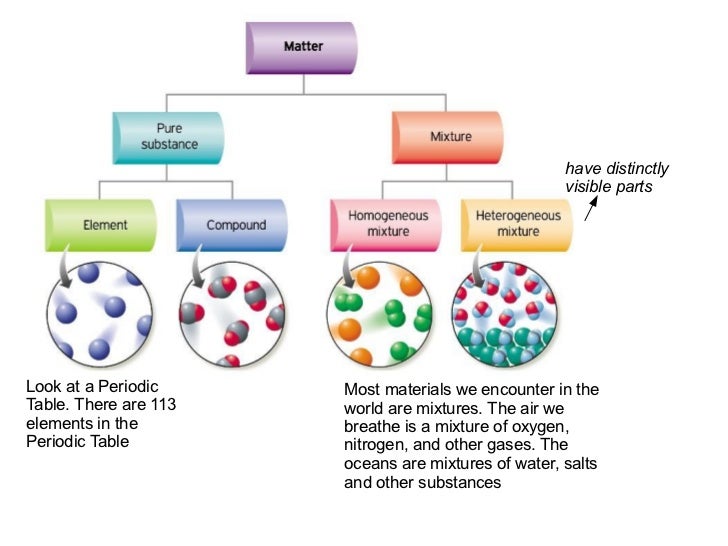
7 Snc Pure Substances Mixtures Lessons Tes Teach

Mixtures Pure Substances Tape In

Give Three Reasons To Show That Air Is A Mixture Not A Compound Science Is Matter Around Us Pure Meritnation Com

Oneclass Assign Each Of The Following Descriptions Of Matter To One Of The Following Categories Het

What Are The Types Of Pure Substances Compounds Elements Videos
Http Sjutsscience Weebly Com Uploads 3 7 4 5 Classifiying Matter Ws Key Pdf
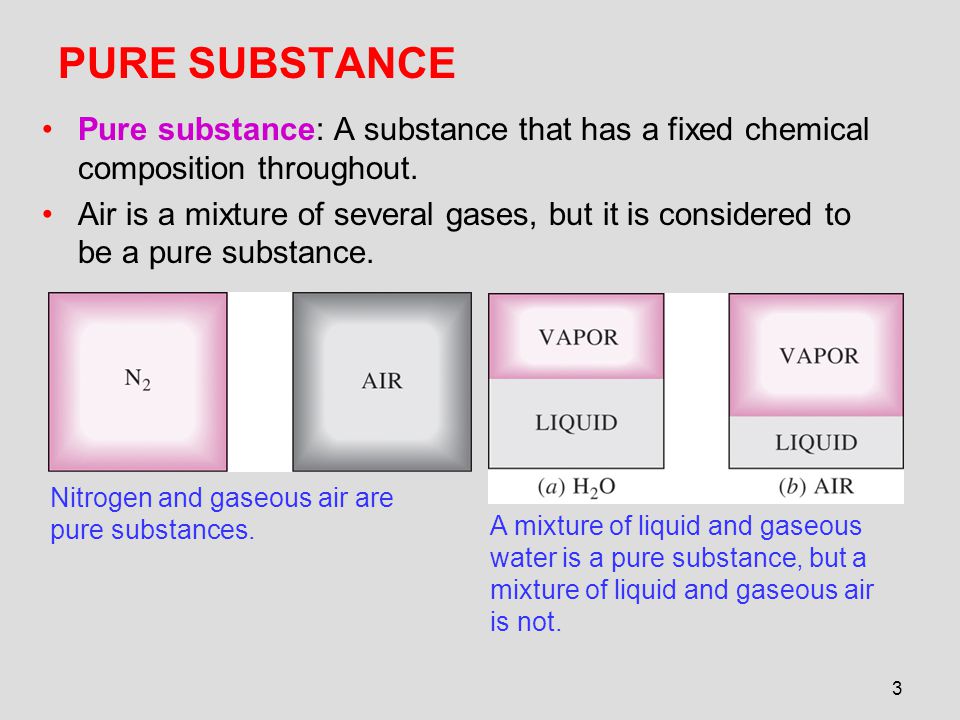
Chapter 3 Properties Of Pure Substances Ppt Video Online Download

What Is A Pure Substance Teaching Resources



